Modelling & Simulation Core
The LEAP Modelling and Simulation Core is a service that supports both selection of the most promising drug candidates and development of drug delivery strategies. It provides state-of-the-art support to investigators working on preclinical and clinical development of LA/ER ARV formulations, in an effort to streamline the development of LA/ER therapies. Investigators can freely engage with the Modelling and Simulation Core as Users, and access predictive models to simulate the pharmacokinetics of LA candidates.
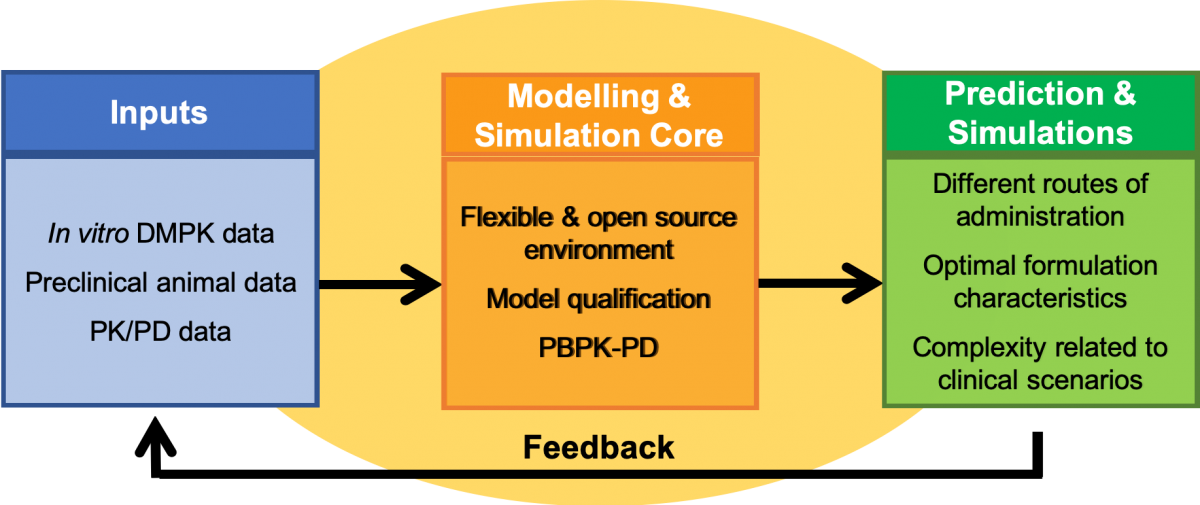 Modelling Core Approach
Modelling Core Approach
In vivo drug disposition is considered through four phases: absorption, distribution in tissues and organs, metabolism, and excretion (also known as ADME). Mathematical equations have been developed to describe processes influencing drug ADME and the resulting pharmacokinetics. Each process is described in a concentration-dependent and time-dependent manner, through physiologically-based pharmacokinetic (PBPK) models based on current information about organ function in human populations. As such, anatomical, physiological, and molecular processes—along with physicochemical properties of the drug and formulation—are captured to define drug distribution, integrating patient-specific factors. Through the inclusion of additional modules to represent other routes of administration, it is possible to simulate across other modalities that are being investigated (eg, subcutaneous implants or systemic intracellular depots). Such simulations can be optimized for multiple delivery routes or strategies and used to generate dose and release rate combinations to inform target product profiles for LA/ER ARV development.
The prospective application of this information to preclinical formulation development and early clinical Phase I study results is intended to improve the efficiency of development by identifying the most promising drug candidates and formulations. The LEAP Modelling and Simulation Core has developed flexible PBPK models that are open-source and can be integrated with novel modules to simulate specific formulation and drug characteristics. Additionally, the modelling strategy can be further integrated into PK/PD approaches for disease specific consideration for each therapeutic area (TB, HIV, and hepatitis), providing a comprehensive framework for evaluating different administration strategies.
Accessing the Modelling and Simulation Core
Users of the Modelling and Simulation Core can discuss potential applications by contacting Doaa Ahmed Mohamed at D.Ahmed-Mohamed@liverpool.ac.uk and by completing the required Submission Form, linked below. The Submission Form calls for details about the drug candidate, target disease and use case, special handling/stability, expected route of delivery, device technology (if applicable), type of formulation and physicochemical properties, and supporting pharmacology data.
A stage-based approach will be used to evaluate the strategy of the modelling approach. Information related to formulation(s) and the drugs of interest to support the development of reliable models are evaluated by Modelling and Simulation Core staff and, if key parameters are lacking or unknown, the Core staff provides support to complete the submission and apply modelling approaches for the generation of input data (eg, using QSARs or suggesting the design of additional experiments).
Projects are selected based on a ranking of scientific merit by Core staff and Executive Committee members, using available supporting data, and based on likelihood of success. Final decisions are directed by the Executive Committee.
Integration of Modelling and Simulation in the LEAP Process
The Modelling and Simulation Core also develops in silico models as part of the LEAP Process to evaluate existing and experimental drug candidates that meet clinical and public health needs that are identified through the landscape analysis. This approach aims to identify the most promising drug candidates and drug delivery technologies to support rational development of future initiatives.
DOWNLOAD SUBMISSION FORM
Please submit the completed form by email to Doaa Ahmed Mohamed at D.Ahmed-Mohamed@liverpool.ac.uk

